Neat Tips About How To Tell If A Compound Is Ionic Or Covalent

Thus, the periodic table can help us recognize ionic compounds.
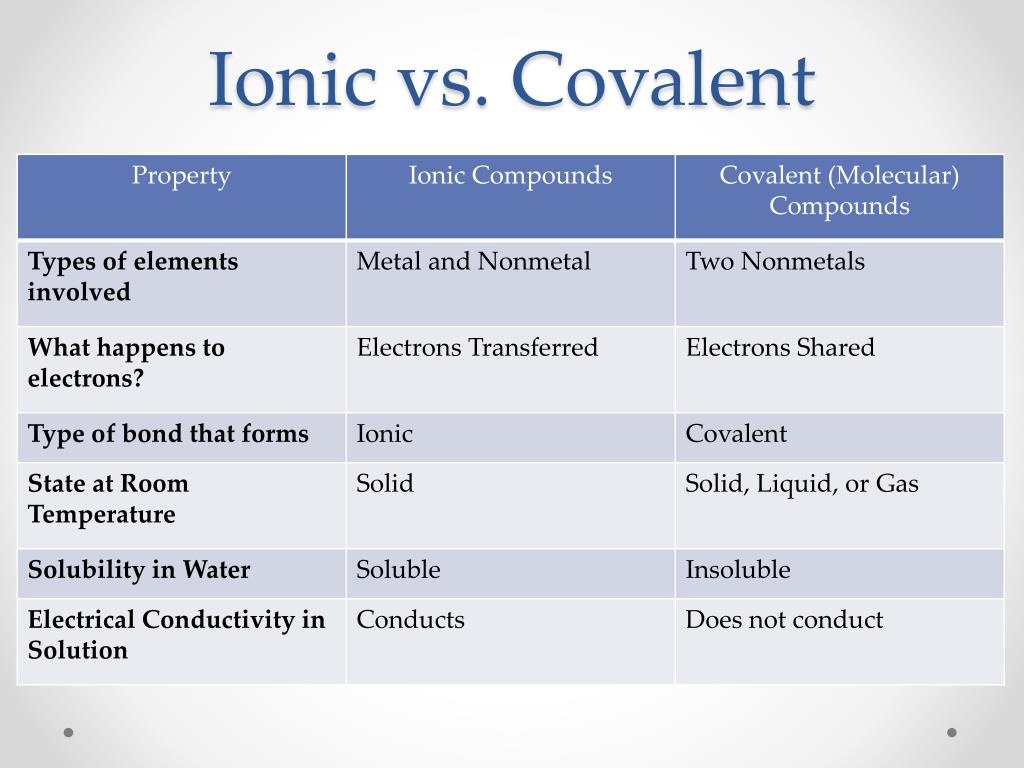
How to tell if a compound is ionic or covalent. Metal + nonmetal to figure out whether an element is a metal or a nonmetal, look at the periodic table. The periodic table can help us recognize many of the compounds that are. Learn the definition, formula, and examples of ionic and covalent compounds.
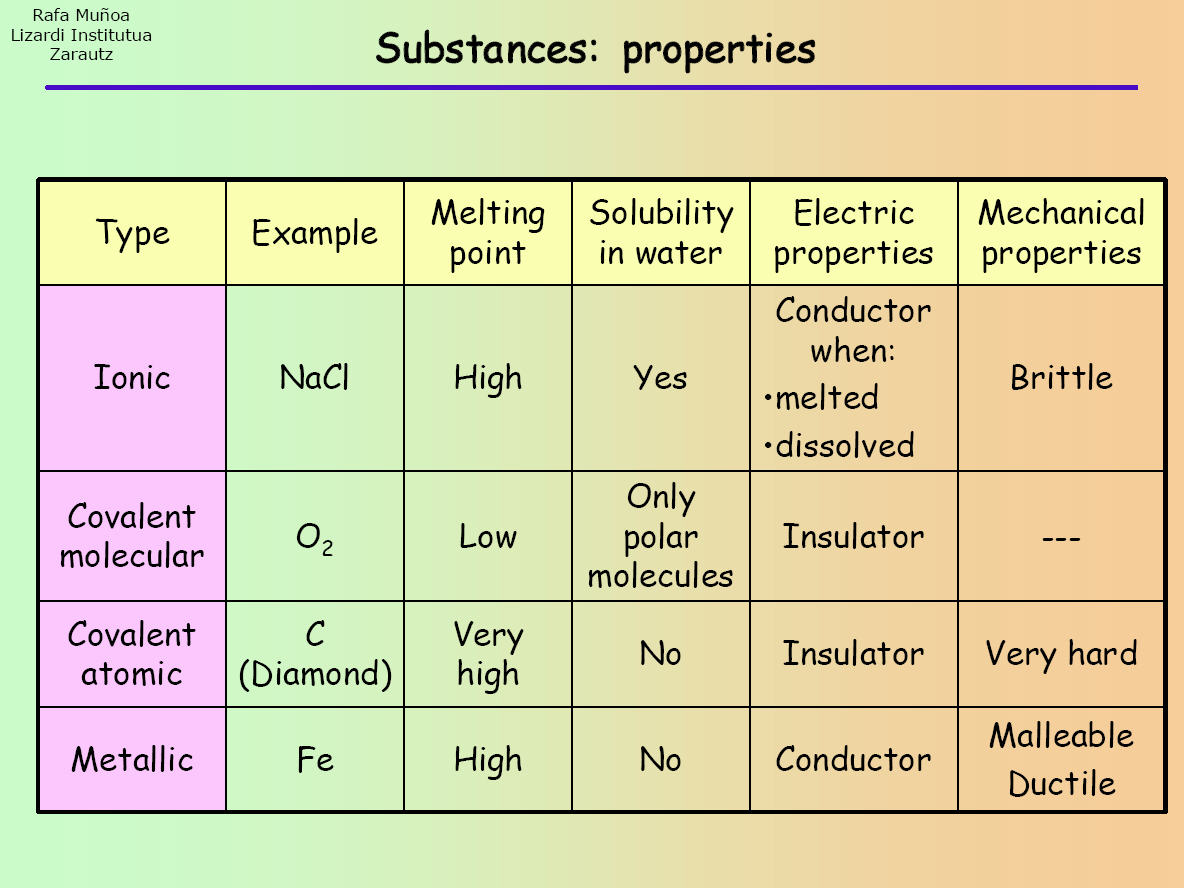
For example, cabr2 contains a metallic. There is a point in fajans' rules that the compound which has more charge number that compound will show covalent character. It explains that ionic, covalent, and metallic compounds have different.
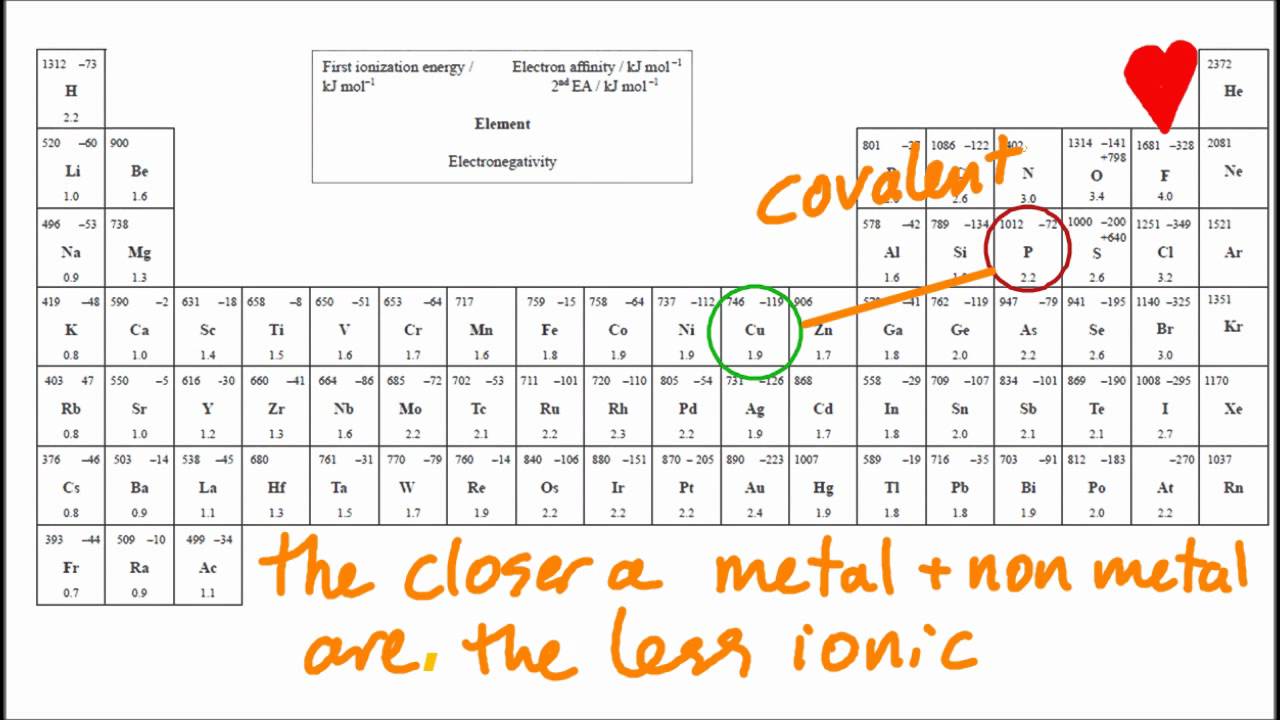
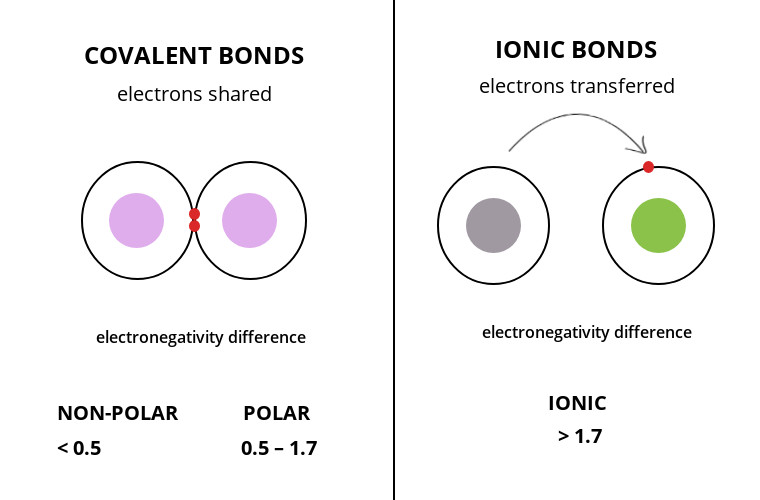
When both ionic and covalent bonding occurs in a compound, the ionic portion is almost always between the cation and anion of the compound. Calculate the difference between the electronegativities δ. Compounds between metal and nonmetal elements are usually ionic.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. For naming these compounds, watch the naming videos!ionic: In contrast, atoms with the same.
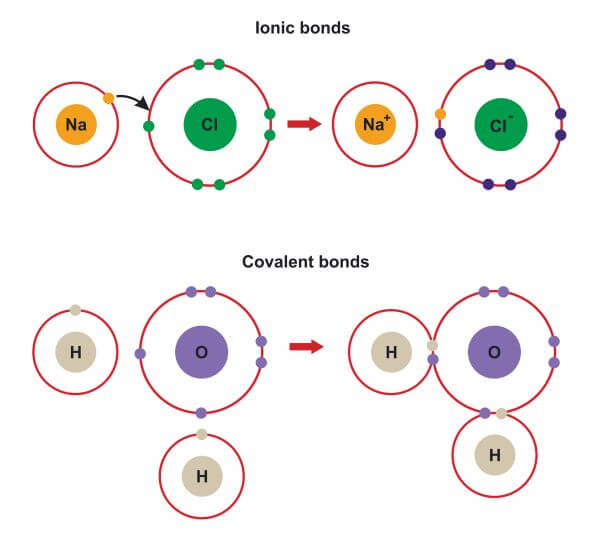
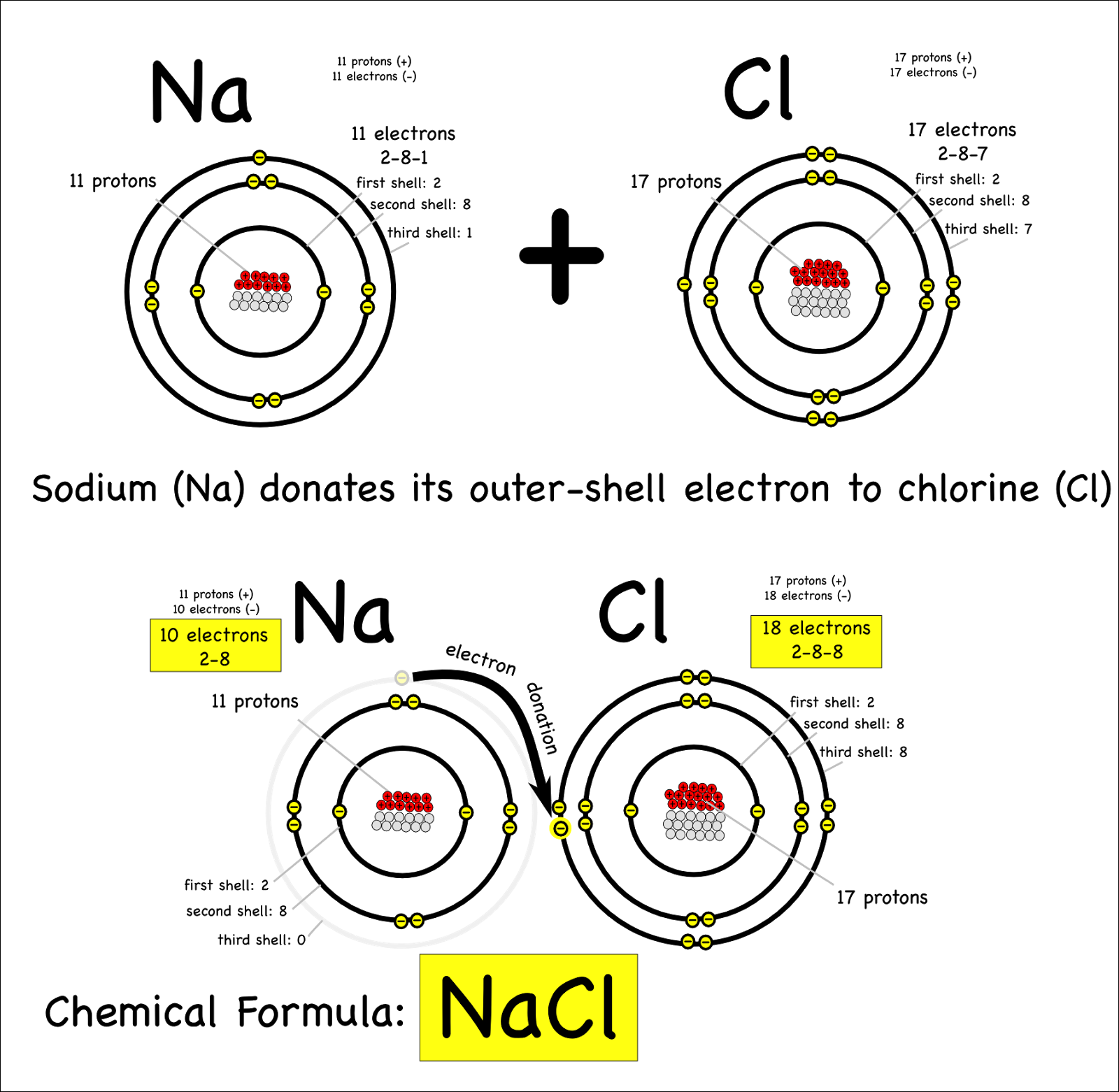
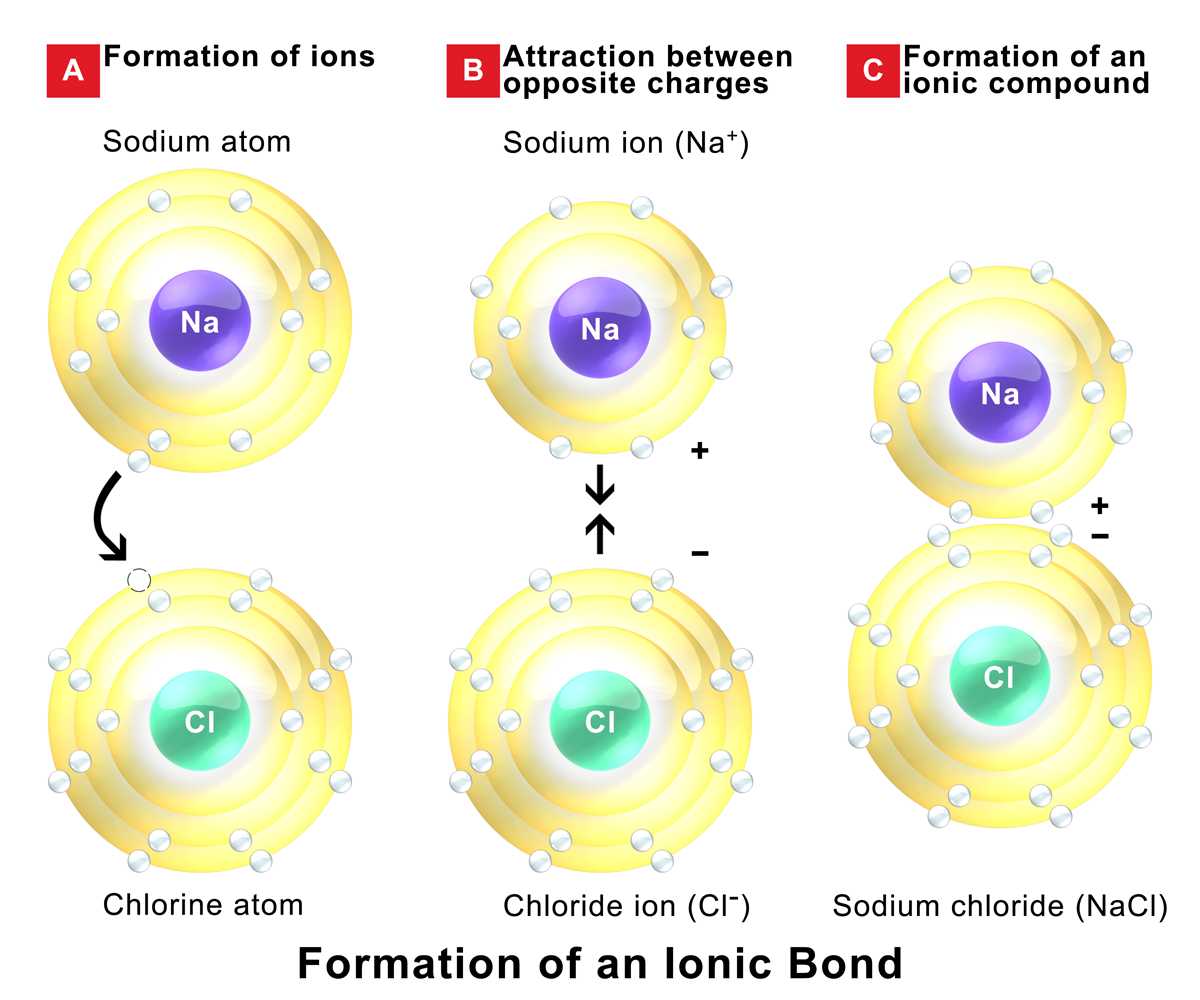
Find out how to identify the formation of ionic and covalent bonds. Left of the metalloid staircase), it is a metal and the compound is ionic. when. In alclx3 a l c l x 3, al has the.
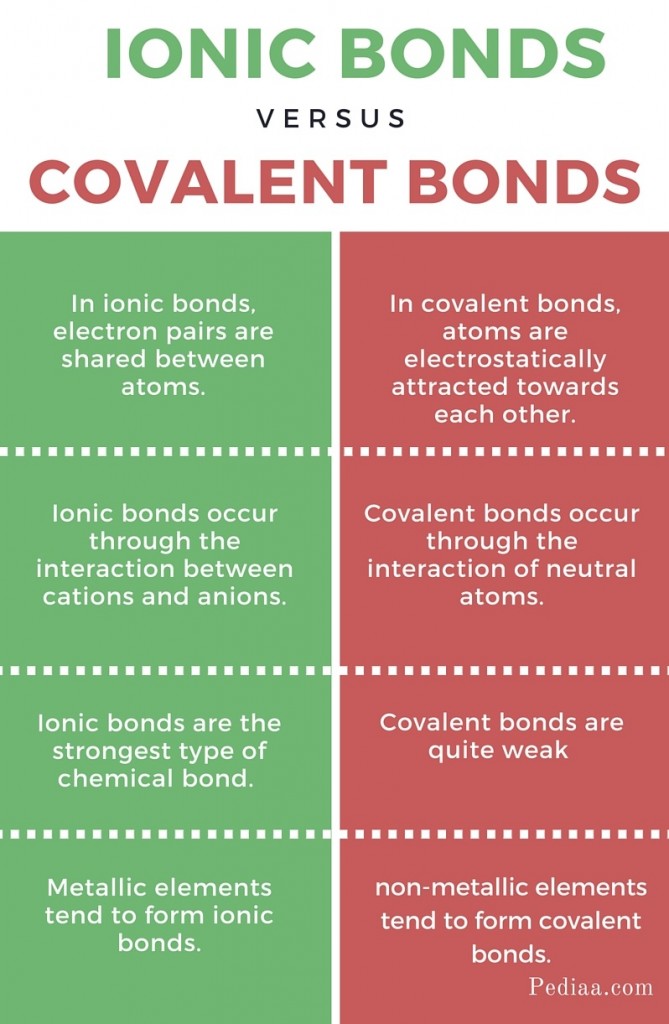
Learn the difference between ionic and covalent bonds with examples, diagrams and electron gain enthalpy. How to determine whether a compound is ionic, covalent, or an acid. However, within the polyatomic phosphate.
Find out how to identify a compound by its formula, group number,. If the first element you see in the compound is found on the left side of the periodic table (i.e. Moles, molecular formulas, and calculating empirical formulas for.
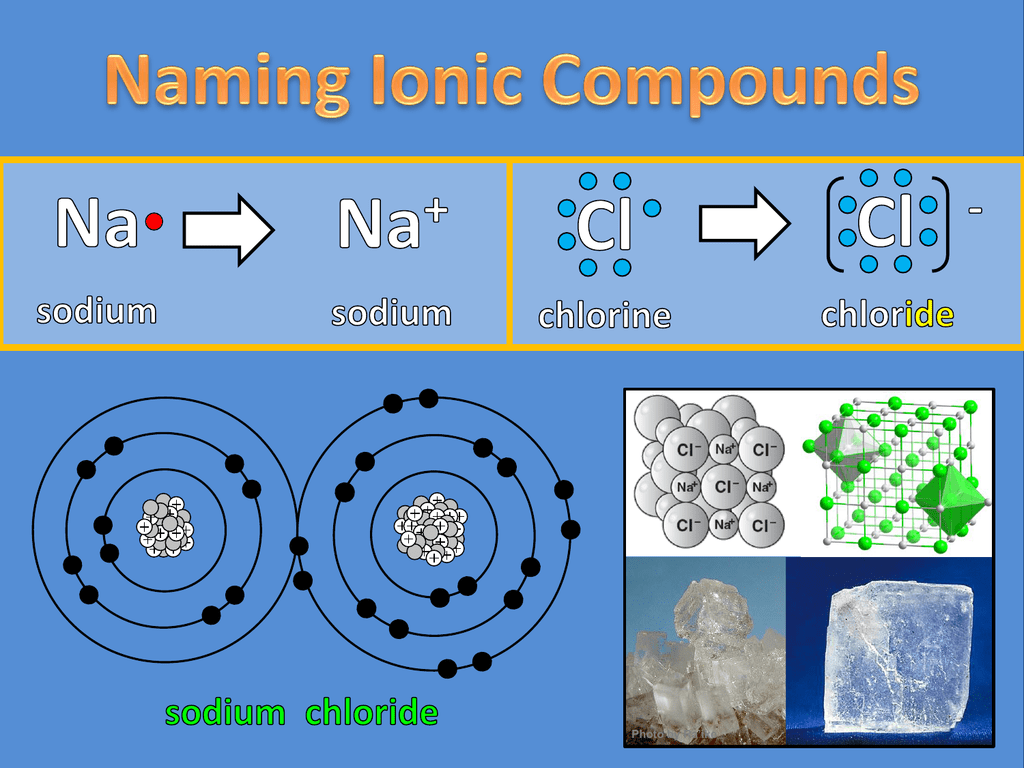
Predicting whether a compound is ionic or covalent. Ionic bonds require at least one electron donor and one electron acceptor. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative charges.
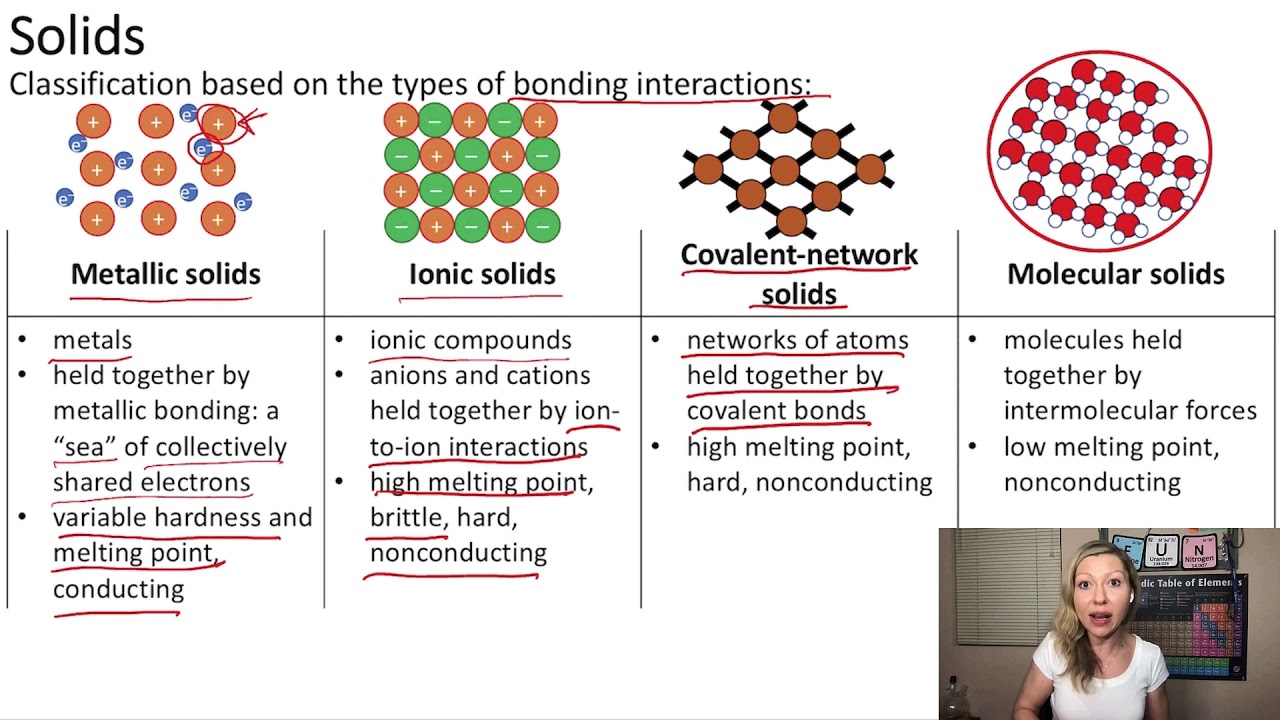
Formula masses of ionic compounds can be determined from the masses of the atoms in their formulas. Identify the electronegativity of the given elements. Almost all the compounds in chemistry can be broadly categorized into ionic and covalent.
A compound that contains ions and is held together by ionic bonds is called an ionic compound. In ionic bonding, atoms transfer electrons to each other. Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic.