Here’s A Quick Way To Solve A Info About How To Obtain Consent

If patients can sign the.
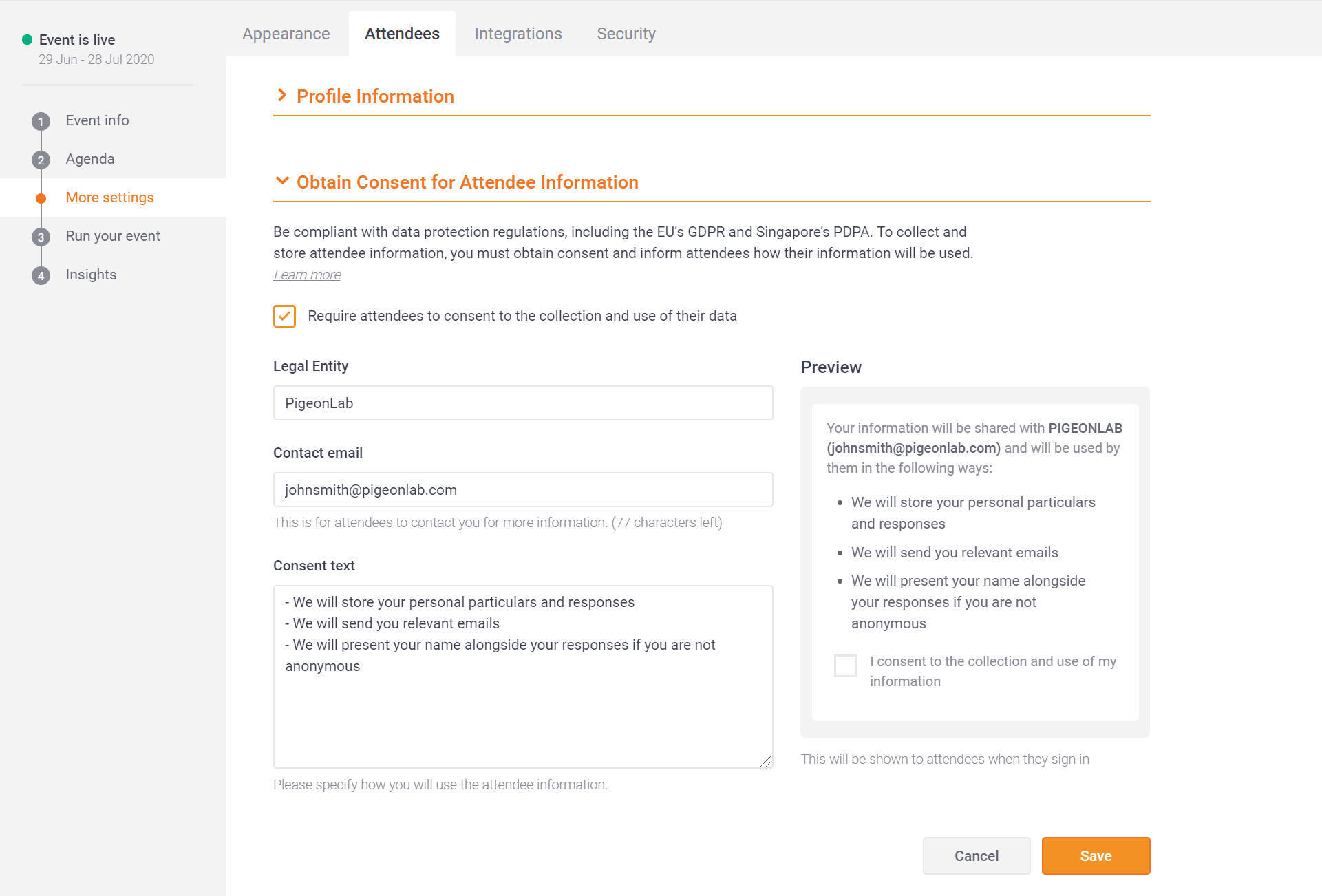
How to obtain consent. All four elements of consent are equally important, namely; This bill, if passed, would force cras to essentially obtain the written instructions of the consumer to release a consumer report to a financial institution, even. The purpose of the research.
Obtaining informed consent from patients participating in clinical research is an important legal and ethical imperative for clinical trial researchers. If you need a signed form, use your patient portal or the mail to get a signature. The process to obtain valid informed consent in healthcare reflects many aspects.
Obtaining genuine informed consent from research participants is best thought of as a process of sharing information and. Consent in medical emergencies for trauma patients. The process of obtaining informed consent.
Methods to gain online or verbal consent. What is the purpose of informed consent? Informed consent is a crucial ethical and legal requirement for research involving human participants.
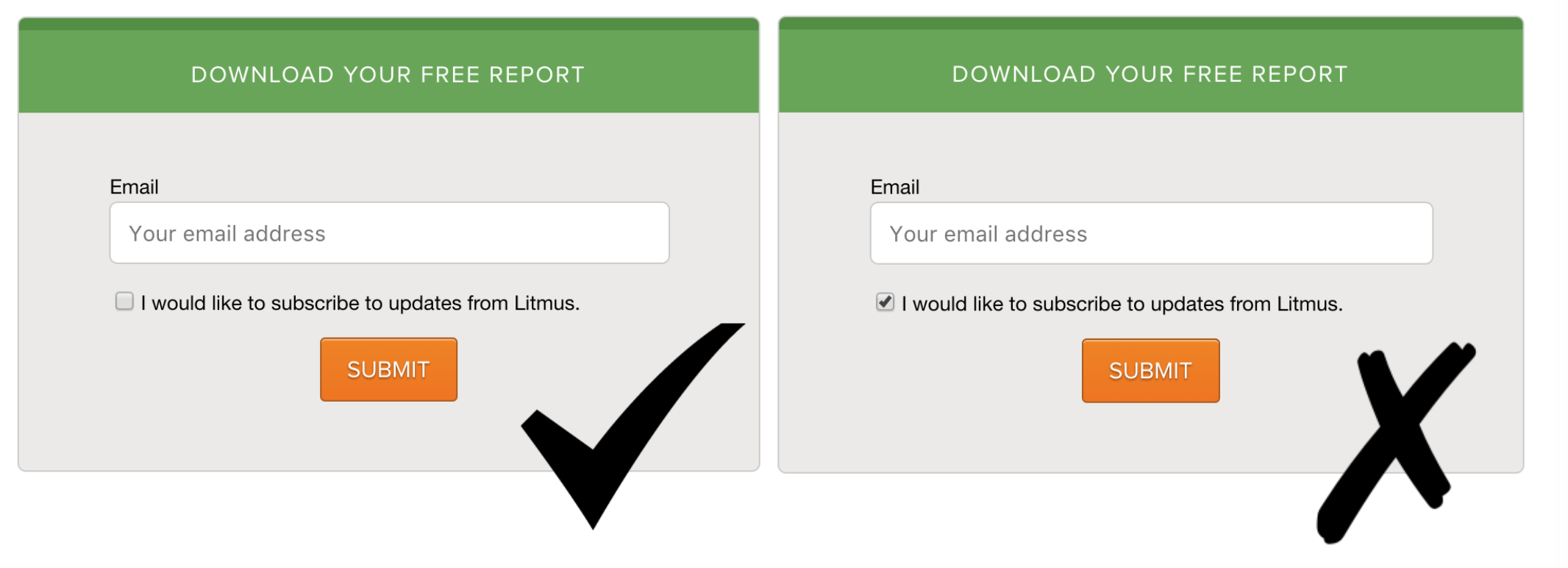
If consent was given online, your records should include the data submitted as. Sample language for online/remote consent, parent permission, and assent. In order that research without consent is considered justifiable, the following three conditions have to be met:
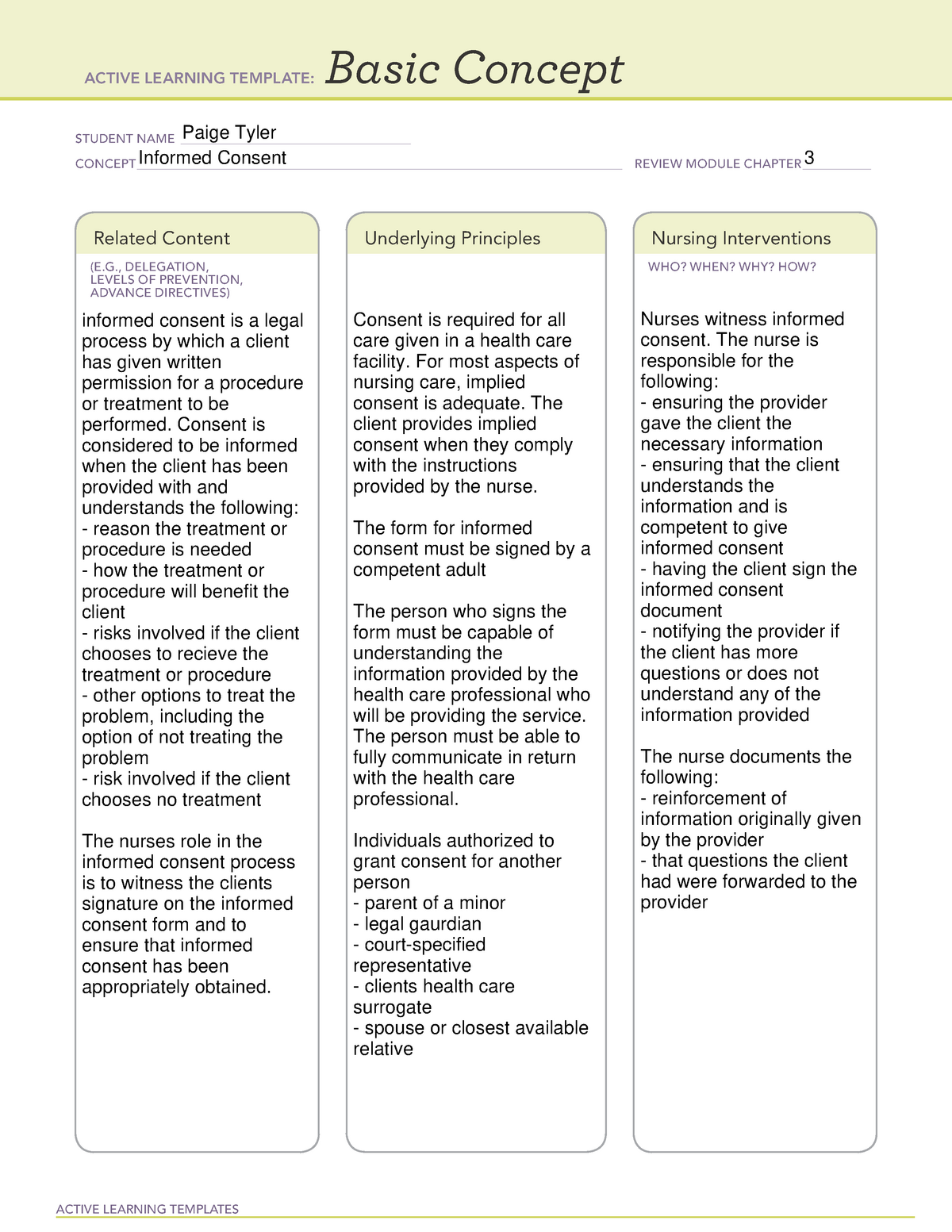
Informed consent has become the primary paradigm for protecting the legal rights of patients and guiding the ethical. In some circumstances, the irb may waive the requirement for documented or written consent and allow researchers to obtain verbal consent. If the irb grants a waiver of.
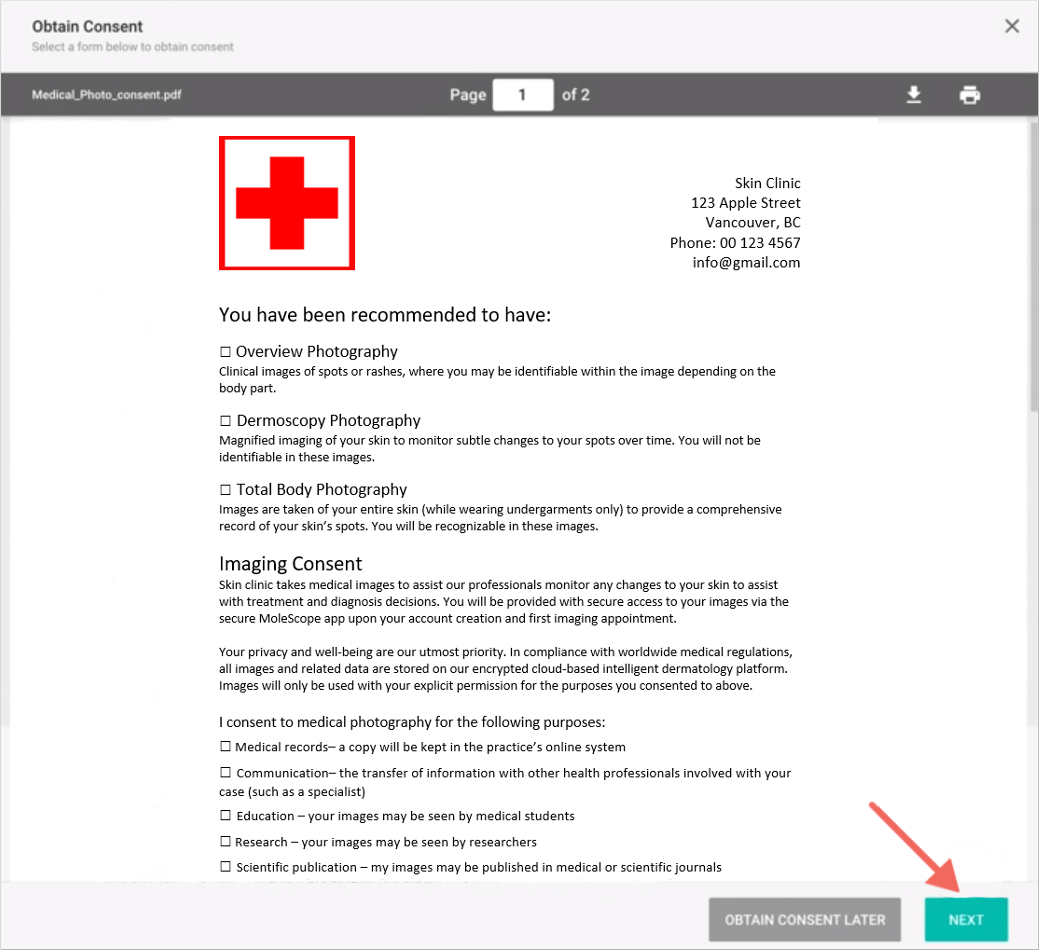
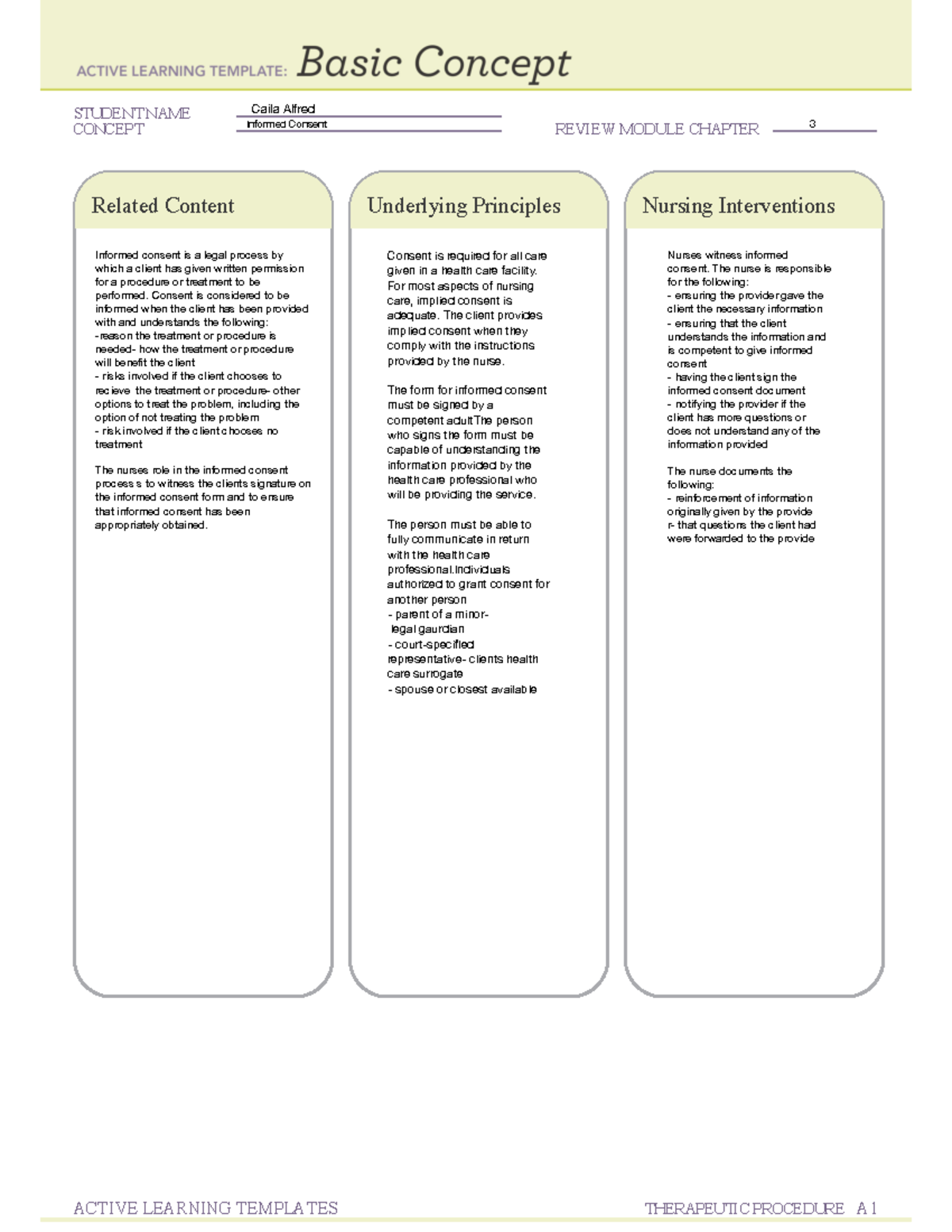
Informed consent is documented by means of a written, signed and dated informed consent form. Consent by a person must be in writing when required by law or by the policies of the state, territory or healthcare organisation where the person is receiving care and treatment. What will happen during the.
Who is doing the research. [dr adib] failed to obtain written consent for the trial and then submit a 'trial consent form' where the patient's sticker was the 'surrogate signature' for the patient. What is informed consent and when, why, and how must it be.
This form is required in the following cases: There are several conditions where it is permissible not to obtain informed consent for medical treatment. Healthcare professionals that take care of the patient must.
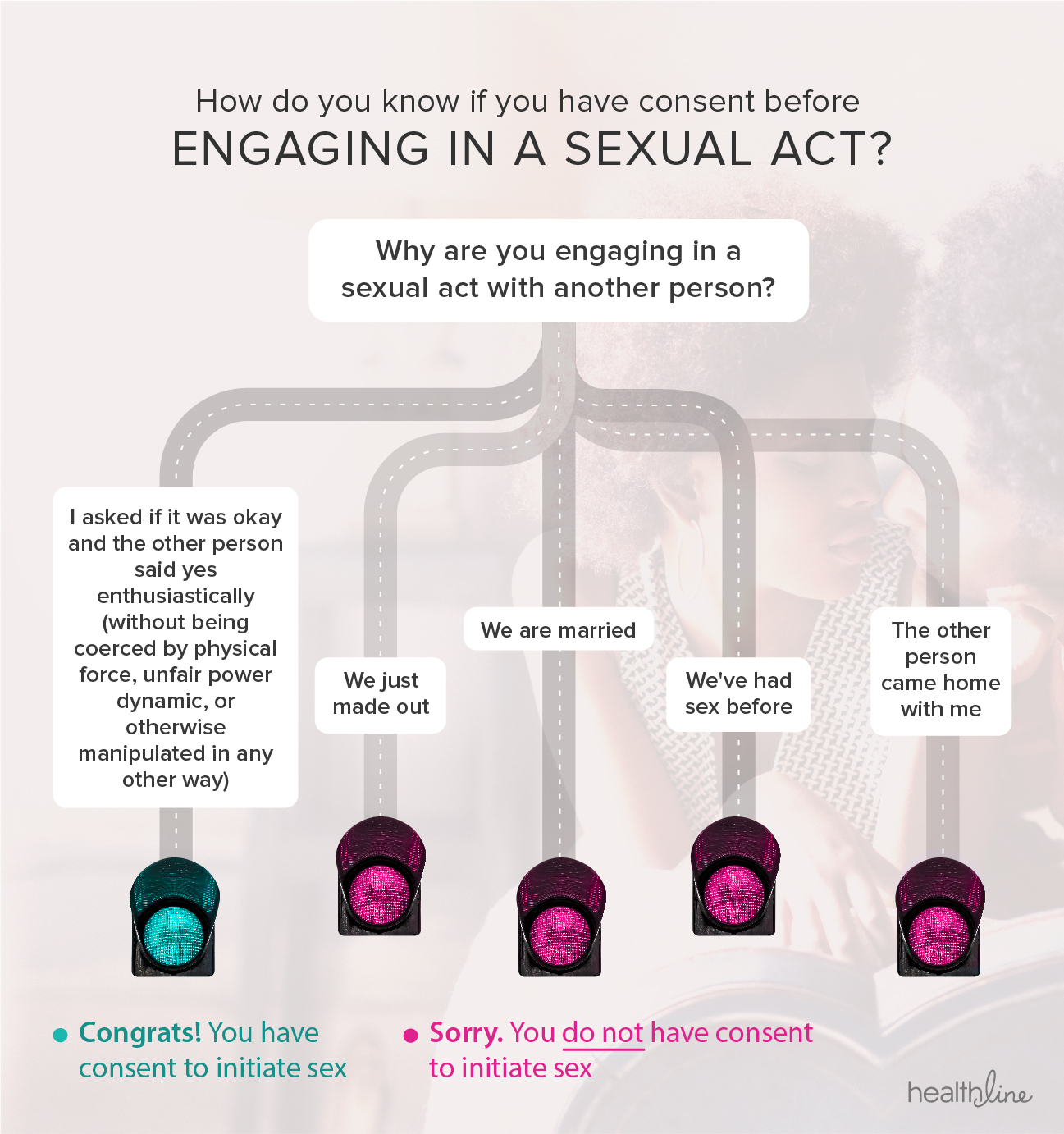
Consent can be given: For written consent, a copy of the relevant document or data capture form. And iv) the ongoing or continuing nature of permission.